Health
Cytokinetics Secures FDA Approval for Heart Drug Myqorzo

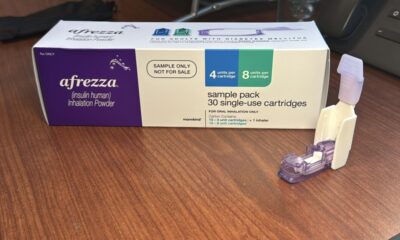
Cytokinetics has achieved a significant milestone by securing its first drug approval from the U.S. Food and Drug Administration (FDA). The FDA granted clearance on January 5, 2024 for Myqorzo, a medication designed to treat patients suffering from obstructive hypertrophic cardiomyopathy, a genetic heart condition that causes the heart muscle to thicken.
Myqorzo will be available to patients starting in late January, although the company has not yet disclosed the pricing details. This approval marks the culmination of a 27-year journey for Cytokinetics, which has focused on developing innovative therapies for serious cardiovascular conditions.
Market Competition and Potential Revenue
Cytokinetics will enter a competitive market, facing off against a similar treatment from Bristol Myers Squibb. That drug, which received approval in 2022, has quickly gained traction, generating more than $1 billion in annual sales. This figure underscores the substantial demand for effective treatments for obstructive hypertrophic cardiomyopathy, highlighting the potential market for Myqorzo.
The approval of Myqorzo is expected to provide patients with a new option for managing their condition. The therapeutic landscape for this heart disorder has evolved, and the introduction of Myqorzo could lead to improved outcomes for many individuals affected by this illness.
Cytokinetics’ CEO, Robert I. Blum, expressed optimism regarding the approval, stating that the company is dedicated to delivering therapies that address unmet medical needs. The launch of Myqorzo aligns with this mission, and the company aims to ensure that patients have access to this new treatment as soon as possible.
As the healthcare community anticipates the drug’s release, the focus will shift to Myqorzo’s clinical performance and how it compares to existing treatment options. With the increasing prevalence of obstructive hypertrophic cardiomyopathy, the demand for effective therapies continues to rise, making Myqorzo’s entry into the market a significant development.
In conclusion, the FDA’s approval of Myqorzo represents a pivotal moment for Cytokinetics and its commitment to advancing heart health. The upcoming months will reveal the drug’s impact on patients and its potential to carve out a substantial share of the market for heart disease treatments.
-

 Science1 month ago
Science1 month agoNostradamus’ 2026 Predictions: Star Death and Dark Events Loom
-

 Technology2 months ago
Technology2 months agoOpenAI to Implement Age Verification for ChatGPT by December 2025
-

 Technology6 months ago
Technology6 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health4 months ago
Health4 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health5 months ago
Health5 months agoAnalysts Project Stronger Growth for Apple’s iPhone 17 Lineup
-

 Technology4 months ago
Technology4 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Education5 months ago
Education5 months agoHarvard Secures Court Victory Over Federal Funding Cuts
-

 Health5 months ago
Health5 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology6 months ago
Technology6 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology7 months ago
Technology7 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology7 months ago
Technology7 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Science3 months ago
Science3 months agoStarship V3 Set for 2026 Launch After Successful Final Test of Version 2