Health
Researchers Uncover Mechanism Behind Immunotherapy Resistance in Tumors
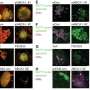
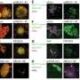
Recent research has identified a significant barrier to the effectiveness of solid tumor immunotherapy, particularly in cases of breast cancer. A study conducted by a team led by Erik Nelson at the Cancer Center at Illinois (CCIL) has revealed that the protein ABCA1 plays a crucial role in regulating the immune response against tumors. This discovery sheds light on why certain immunotherapies, despite their successes, fail to work for many patients.
Immunotherapy leverages the body’s immune system to combat cancer, with one of the most effective strategies being immune checkpoint blockade. This method functions by removing the molecular “brakes” on T cells, enabling them to identify and destroy cancer cells more effectively. However, in numerous cases, particularly with solid tumors such as breast cancer, these therapies have shown limited effectiveness.
Understanding the underlying mechanisms of this resistance is essential. The research team at CCIL focused on the role of the ABCA1 protein, which appears to inhibit the activity of T cells within the tumor microenvironment. The study found that elevated levels of ABCA1 can significantly dampen the immune response, thereby allowing tumors to evade detection and destruction by T cells.
Details of the Research Findings
The research team utilized a combination of laboratory experiments and clinical data to substantiate their findings. They discovered that by inhibiting the function of ABCA1, T cells demonstrated a markedly enhanced response to immunotherapy treatments. This suggests that targeting ABCA1 could potentially improve the efficacy of existing therapies for solid tumors. The study emphasizes the need for further investigation into the modulation of ABCA1 as a strategy to bolster the immune response in cancer patients.
Nelson remarked on the importance of these findings, stating, “Our research provides crucial insights into why certain patients do not respond to immunotherapy. By identifying ABCA1 as a significant player in immune evasion, we open the door for new therapeutic strategies that could enhance treatment outcomes.”
The implications of this research extend beyond breast cancer, as similar mechanisms may be at play in various solid tumors. This discovery could pave the way for new treatment protocols that incorporate ABCA1 targeting alongside traditional immunotherapies.
Future Directions
As the research progresses, the team at CCIL plans to explore potential inhibitors of ABCA1 that could be utilized in clinical settings. Their goal is to develop a combination therapy that not only enhances T cell activity but also improves patient outcomes across different types of solid tumors.
With ongoing advancements in cancer research, the hope remains that these findings will contribute to a broader understanding of immunotherapy resistance and eventually lead to more effective treatments for patients facing challenging diagnoses. The fight against cancer continues to evolve, and studies like this one are vital in steering the direction of future therapies.
The ongoing work by Nelson and his team exemplifies the commitment to unraveling the complexities of cancer immunology and improving therapeutic strategies for those affected by this disease.
-

 Science1 month ago
Science1 month agoNostradamus’ 2026 Predictions: Star Death and Dark Events Loom
-

 Technology2 months ago
Technology2 months agoOpenAI to Implement Age Verification for ChatGPT by December 2025
-

 Technology7 months ago
Technology7 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health5 months ago
Health5 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health5 months ago
Health5 months agoAnalysts Project Stronger Growth for Apple’s iPhone 17 Lineup
-

 Technology5 months ago
Technology5 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Education5 months ago
Education5 months agoHarvard Secures Court Victory Over Federal Funding Cuts
-

 Health5 months ago
Health5 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Science2 months ago
Science2 months agoBreakthroughs and Challenges Await Science in 2026
-

 Technology7 months ago
Technology7 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology6 months ago
Technology6 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Science4 months ago
Science4 months agoStarship V3 Set for 2026 Launch After Successful Final Test of Version 2