Science
FDA to Decide on Biohaven’s Controversial Drug for SCA

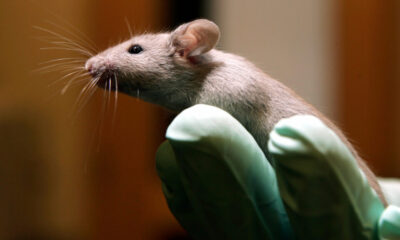
The Food and Drug Administration (FDA) is set to announce its decision in mid-November 2023 regarding Biohaven Pharmaceuticals’ treatment for spinocerebellar ataxia (SCA), a rare neurodegenerative disease that significantly impacts patients’ quality of life. This decision is particularly crucial as it tests the limits of the agency’s regulatory flexibility under its current leadership.
Biohaven’s application for approval has sparked considerable debate, primarily due to the controversial nature of its data package. Investors and stakeholders are watching closely, with sentiments varying widely about the likelihood of approval. A Biohaven shareholder expressed their views, stating, “This is a really tough call. I’d say approval is a 50-50 shot, at best.” Such uncertainty has become a common theme surrounding FDA decisions, particularly in cases involving complex and poorly understood conditions like SCA.
Impact of Regulatory Decisions on Biohaven
The upcoming FDA ruling could have significant implications for Biohaven, both financially and in terms of its reputation within the pharmaceutical industry. The company’s treatment aims to address the symptoms of SCA, which affects coordination and balance, leading to severe disability. As the FDA evaluates the available data, the agency faces the challenge of balancing the need for rigorous scientific scrutiny with the urgency for effective treatments for rare diseases.
Analysts have noted that the uncertainty surrounding this decision reflects broader trends in the regulatory landscape, where the FDA’s approach to flexibility is under increasing scrutiny. This particular case highlights the tension between the need for thorough evaluation and the pressing demand for new therapies for patients facing debilitating conditions.
As the mid-November deadline approaches, stakeholders will be keenly observing not only the FDA’s decision but also the rationale behind it, which could set a precedent for future drug approvals in similar contexts. With the stakes high for both Biohaven and patients suffering from SCA, the outcome of this ruling will resonate throughout the industry.
In summary, the FDA’s ruling on Biohaven’s treatment for SCA will serve as a critical benchmark for regulatory flexibility and decision-making in the field of rare diseases. As the pharmaceutical landscape evolves, the implications of this decision may extend far beyond Biohaven’s immediate future.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle