Technology
Small-Scale Chromatography Advances mAb Production and Validation
The biopharmaceutical industry is set to benefit significantly from advancements in small-scale chromatography models, particularly in the production and validation of monoclonal antibodies (mAb). During a recent webinar hosted by GEN on November 6, 2025, expert panelist Matt Taylor, a Senior Process Development Associate at KBI Biopharma, outlined how pre-packed chromatography columns can streamline the mAb purification process.
As the demand for therapeutic proteins continues to rise, traditional column packing and validation methods have become increasingly cumbersome, leading to bottlenecks in downstream manufacturing workflows. Taylor emphasized the need for more agile validation methods that allow manufacturers to operate efficiently while adhering to stringent regulatory standards.
Transforming Downstream Processes
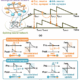
Taylor’s presentation focused on the feasibility of using pre-packed columns as scaled-down models for mAb purification, aligning with the ICH Q5A guidelines. He shared compelling data indicating that these pre-packed formats can provide performance equivalency to large-scale operations. By adopting such technologies, manufacturers can significantly reduce preparation time, enabling teams to concentrate on activities that add greater value.
One of the key advantages highlighted was the impact on viral clearance validation. Taylor noted that pre-packed columns deliver comparable viral clearance performance, even when using aged resin. This capability not only meets regulatory requirements but also minimizes preparation and experimental cycle times, which are crucial in a fast-paced industry.
Enhancing Operational Efficiency
Eliminating the need for column packing and validation steps offers operational and productivity benefits that cannot be overlooked. By streamlining these processes, development resources can be redirected towards more strategic initiatives, ultimately accelerating timelines for bringing therapeutics to market.
The webinar concluded with a live Q&A session, allowing attendees to engage directly with Taylor and gain further insights into the implications of these advancements in chromatography technology.
In summary, the integration of small-scale chromatography models represents a significant leap forward in the biopharmaceutical field, promising enhanced efficiency and compliance in mAb production processes. As companies like KBI Biopharma lead the way in adopting these innovative technologies, the potential for improved therapeutic development becomes increasingly apparent.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle