


On February 24, 2026, the U.S. Food and Drug Administration (FDA) announced the introduction of new standards for its Produce Regulatory Program Standards (PRPS). This initiative,...



On February 17, 2026, California introduced Assembly Bill 2034 (AB 2034), a significant legislative effort aimed at enhancing the safety of food ingredients classified as generally...



The Food and Drug Administration (FDA) has introduced a new regulatory framework aimed at facilitating the approval of personalized gene editing treatments. This initiative, termed the...



A significant concern has emerged regarding a multistate outbreak of botulism associated with infant formula produced by New York-based ByHeart. The Centers for Disease Control and...



A multistate outbreak of botulism has been associated with infant formula products manufactured by New York-based ByHeart. The outbreak may have originated from whole milk powder...
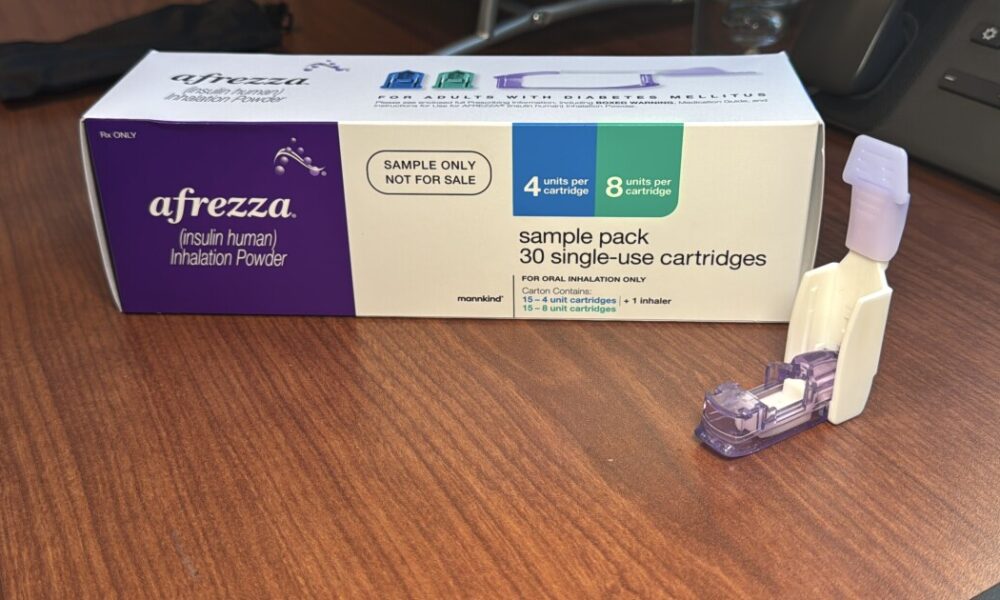
Managing diabetes has evolved significantly, and one of the latest advancements is the introduction of inhaled insulin. Afrezza, approved by the FDA for adults with type...



The U.S. Food and Drug Administration (FDA) has announced a recall of the dietary supplement Vitality Plus, citing significant health risks that could lead to life-threatening...



A recent study indicates that drugs approved under the U.S. Food and Drug Administration’s (FDA) Breakthrough Therapy Designation may carry heightened safety risks. Research conducted by...



Enveda Biosciences, based in Boulder, has received approval from the U.S. Food and Drug Administration (FDA) to initiate clinical trials for its drug candidate, ENV-6946, aimed...



Pentixapharm Holding AG has received encouraging feedback from the U.S. Food and Drug Administration (FDA) regarding its planned Phase 3 PANDA study. The formal written minutes...