Science
Kiran Musunuru Unveils Future of Gene Therapy at ESGCT Conference
The annual conference of the European Society of Gene & Cell Therapy (ESGCT) kicked off in Seville this week, showcasing significant advancements in gene therapy. A key highlight was a presentation by Kiran Musunuru, MD, PhD, from the Perelman School of Medicine at the University of Pennsylvania, who discussed the progress made since the groundbreaking treatment of infant KJ Muldoon, also known as “Baby KJ.” This update marks a pivotal moment in the field of N-of-1 gene editing therapies.
Musunuru shared insights from the past year, emphasizing the need for personalized approaches in treating rare diseases. He noted that his research spans from rare genetic disorders to cardiovascular disease, which claims approximately 18 million lives annually. Musunuru’s lab first achieved a significant milestone in 2014 by using CRISPR-Cas9 technology to target the PCSK9 gene for cardiovascular disease treatment. This work led to the founding of Verve Therapeutics, which was acquired by Lilly in July 2025.
In collaboration with Rebecca Ahrens-Niklas, MD, PhD, from the Children’s Hospital of Philadelphia, Musunuru focused on patients with hereditary urea cycle disorders. These conditions often lead to dangerous levels of ammonia in the bloodstream. Musunuru highlighted the urgency of early intervention, stating that infant mortality rates exceed 50% for affected children. Without timely treatment, ammonia surges can inflict severe brain damage, and liver transplants, typically necessary after 12 months, carry significant risks.
The journey to treat Baby KJ began after his birth, when he was diagnosed with a severe deficiency of the enzyme CPS1, leading to ammonia levels dangerously high—about 30 times above normal. Initially, KJ was admitted to CHOP to await a liver transplant. However, Musunuru and Ahrens-Niklas quickly pivoted to explore a personalized base editing treatment.
Over the subsequent months, the team made rapid progress. They identified KJ’s specific CPS1 mutations and tested various editing constructs using lentivirus-transduced cell lines. Musunuru remarked, “It was all about speed; we couldn’t let perfect be the enemy of the good.” Within four weeks, they identified the most effective guide RNA.
Significant collaboration ensued, engaging experts like Ben Kleinstiver, PhD, from Mass General Hospital, and David Liu, PhD, from the Broad Institute, to enhance the efficiency of the adenine base editor. This teamwork extended to public and private partnerships with organizations such as Aldevron, IDT, and The Jackson Laboratory.
By the time KJ reached four months, the team held a pre-Investigational New Drug (IND) meeting with officials from the U.S. Food and Drug Administration (FDA). Musunuru praised the FDA’s support and commitment to a rapid review process. Toxicology and dosing studies were completed within five months, and by KJ’s six-month birthday, clinical production of the necessary reagents was finalized.
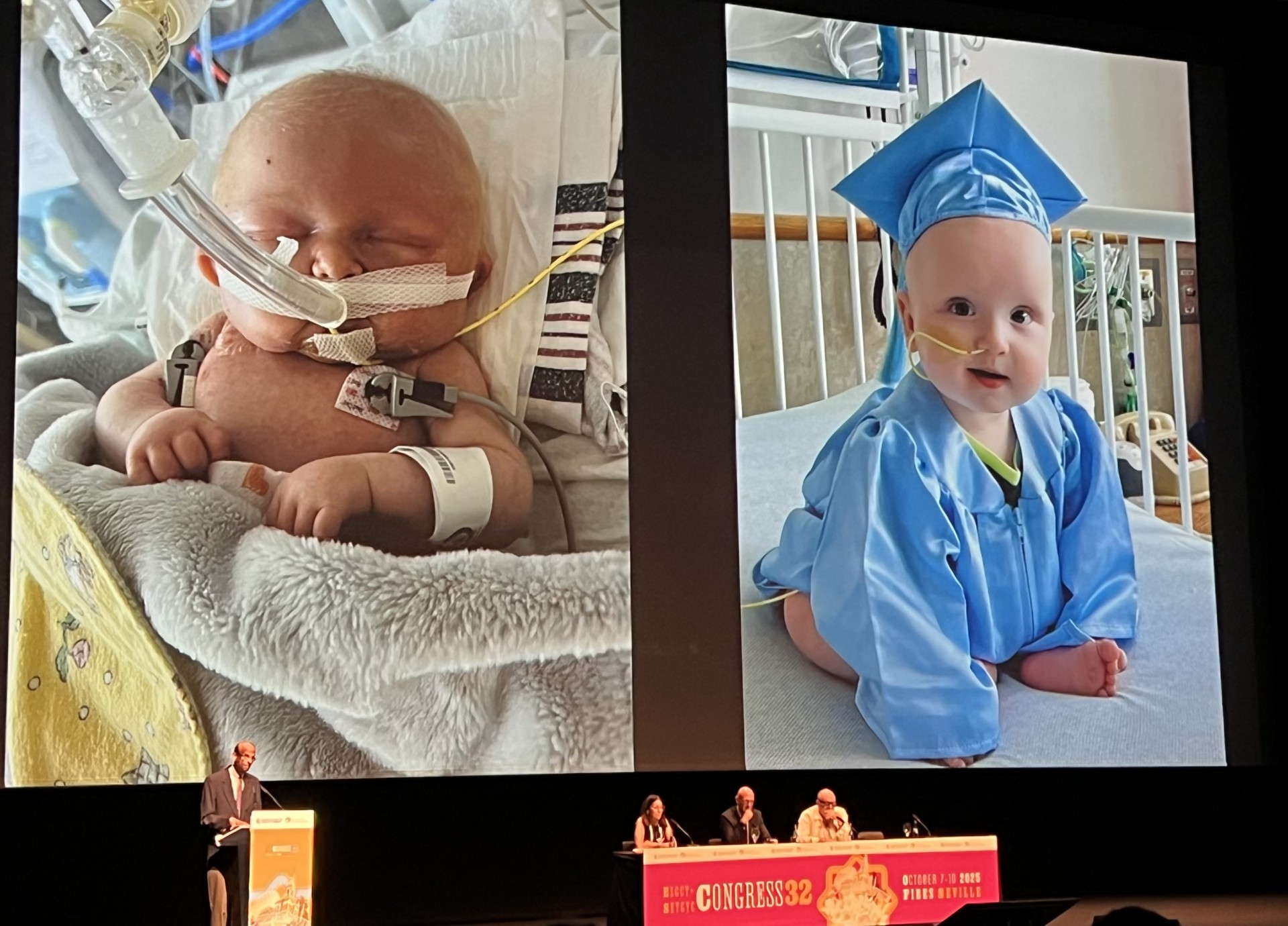
Two weeks later, extensive off-target analysis was conducted using KJ’s genome. The results indicated minimal off-target effects, allowing the team to submit their IND application, which the FDA approved within a week. KJ received his first dose of the lipid nanoparticle and custom base editor when he was seven months old, followed by additional doses at 22 and 62 days later. Aside from mild side effects, KJ exhibited no adverse reactions, and his growth improved notably, rising from the 9th to the 40th percentile.
Musunuru cautioned against labeling the treatment a “cure,” emphasizing the importance of maintaining realistic expectations. He described KJ’s condition as having effectively transformed into a milder variant of the disease, indicating that a liver biopsy is no longer necessary at this stage.
Reflecting on the implications of KJ’s journey, Musunuru suggested a shift from N-of-1 studies to platform trials. He announced plans for a master protocol aimed at a primary IND clinical trial that would encompass multiple urea cycle disorders, including OTC deficiency and arginase deficiency. This framework would allow for gene-specific INDs and amendments based on in vitro data.
Musunuru concluded his presentation by acknowledging KJ’s parents and sharing a poignant “before and after” photograph of KJ’s journey from admission to CHOP to a celebratory moment shortly before his discharge, which included a police escort and a wave of media attention.
The ESGCT conference runs from October 7-10, 2025, in Seville, Spain, continuing to spotlight advancements in gene and cell therapy that hold promise for patients worldwide.
-

 Technology5 months ago
Technology5 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health3 months ago
Health3 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Technology6 days ago
Technology6 days agoOpenAI to Implement Age Verification for ChatGPT by December 2025
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology1 month ago
Technology1 month agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology3 months ago
Technology3 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology5 months ago
Technology5 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology5 months ago
Technology5 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health5 months ago
Health5 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Health3 months ago
Health3 months agoAnalysts Project Stronger Growth for Apple’s iPhone 17 Lineup
-

 Lifestyle5 months ago
Lifestyle5 months agoBelton Family Reunites After Daughter Survives Hill Country Floods