Science
Kiran Musunuru Unveils Future of Gene Therapy at ESGCT 2025
The annual conference of the European Society of Gene & Cell Therapy (ESGCT) commenced in Seville, Spain, this week, showcasing significant advancements in the field of gene therapy. A key highlight was the presentation by Kiran Musunuru, MD, PhD, from the Perelman School of Medicine at the University of Pennsylvania, who discussed the remarkable progress made in the treatment of infant KJ Muldoon, known as “Baby KJ.” This presentation outlined a roadmap for future therapies, building on insights gained from KJ’s treatment over the past year.
During his session, Musunuru reflected on his diverse medical interests, which range from rare diseases to cardiovascular health, the latter responsible for approximately 18 million deaths annually. His lab was notably the first to employ CRISPR-Cas9 technology to target PCSK9, a breakthrough that has since been commercialized by Verve Therapeutics, a company co-founded by Musunuru and Sek Kathiresan, MD. Verve was acquired by Lilly in July 2025.
Challenges in Neonatal Genetic Disorders
In collaboration with Rebecca Ahrens-Niklas, MD, PhD, from the Children’s Hospital of Philadelphia, Musunuru focused on neonatal metabolic disorders, particularly hereditary urea cycle disorders. These conditions can lead to toxic ammonia build-up, with mortality rates exceeding 50% in affected infants. Musunuru emphasized the necessity for personalized therapies in these cases, given the prevalence of unique mutations among patients.
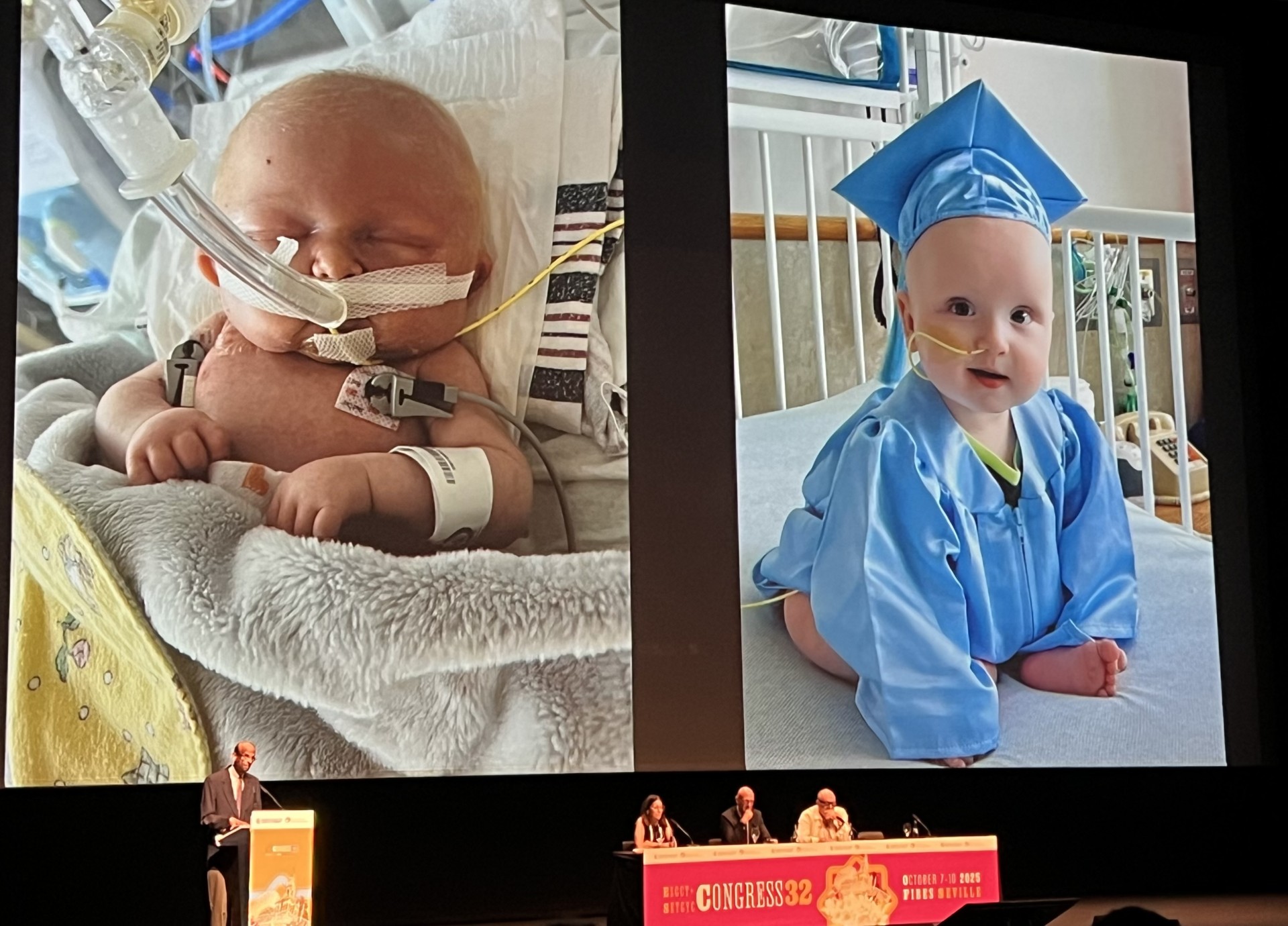
The urgency of addressing these disorders was underscored by KJ’s case. Born with a severe deficiency of the liver enzyme CPS1, KJ exhibited ammonia levels approximately 30 times higher than normal. Initially, he was placed on a waiting list for a liver transplant, but the team pivoted towards developing a tailored gene therapy solution.
Innovative Gene Editing Approach
Musunuru shared that the journey to formulate a viable treatment took several years, culminating last year with KJ’s case. The team identified specific mutations in KJ’s CPS1 gene and employed lentivirus-transduced cell lines to evaluate various gene editing constructs. “It was all about speed; we couldn’t let perfect be the enemy of the good,” Musunuru stated, highlighting their rapid progress in the face of critical time constraints.
Within four weeks of KJ’s birth, a suitable guide RNA was identified. Collaborators in Boston, including Ben Kleinstiver, PhD, and David Liu, PhD, contributed to enhancing the editing efficiency. This collaborative effort included partnerships with organizations like Aldevron and Acuitas Therapeutics, forming a robust public-private network.
In a significant milestone, Musunuru’s team held a pre-investigational new drug (IND) meeting with the U.S. Food and Drug Administration (FDA) four months after KJ’s birth. The FDA expressed strong support for the initiative, committing to a speedy review process. By the six-month mark, the clinical production of necessary reagents was completed, paving the way for treatment.
KJ received the first dose of a lipid nanoparticle-custom base editor at seven months of age, followed by additional doses at intervals. Remarkably, aside from mild coughing and a low-grade fever, KJ experienced no adverse effects from the treatment. Musunuru noted a significant improvement in KJ’s growth trajectory, moving from the 9th percentile to the 40th percentile in weight after his third dose.
Despite the positive outcomes, Musunuru remained cautious, stating, “I avoid using the word cure. We have a responsibility not to exaggerate and give undue hope to the families.” He clarified that while KJ’s condition has improved, it remains a milder form of his disease.
Future Directions in Gene Therapy
The case of KJ represents a paradigm shift in the approach to gene therapy. Musunuru called for a transition from “N-of-1” studies to more comprehensive platform trials. He and his team are developing a master protocol for a primary IND clinical trial that will include patients with various urea cycle disorders, such as OTC deficiency and arginase deficiency. This approach aims to streamline therapies while allowing for adaptations based on specific genetic variations.
As he concluded his presentation, Musunuru expressed gratitude to KJ’s parents and shared a poignant “before and after” photograph of KJ, illustrating the transformation he has undergone. This moment captured not only the advancements in gene therapy but also the hope it brings to families facing similar challenges as ESGCT 2025 continues through October 10.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle