Science
Eli Lilly’s Orforglipron Nears Approval as New Obesity Treatment
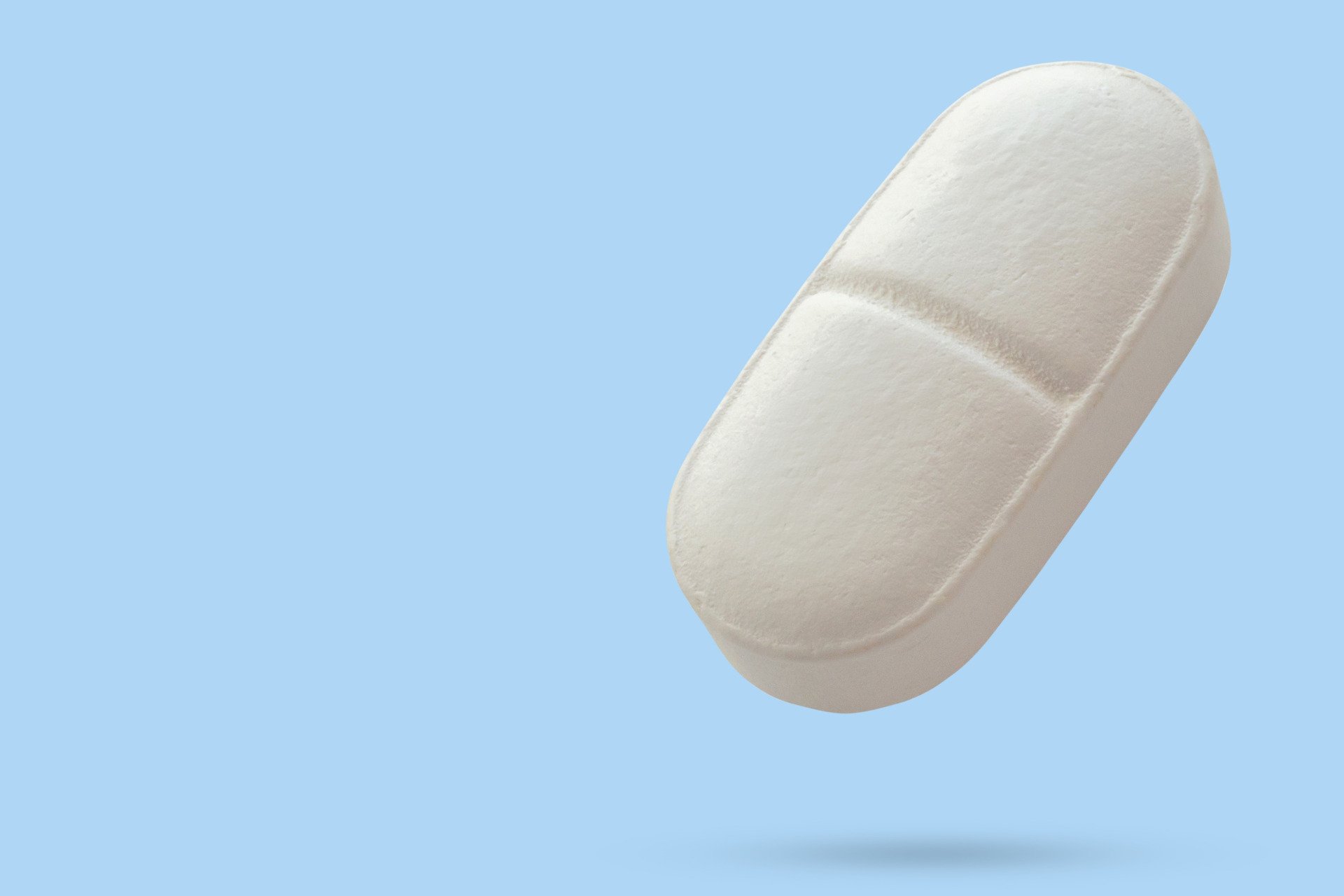
Eli Lilly has reported promising results from its latest Phase III trial of an experimental oral medication designed to combat obesity. The drug, known as orforglipron, has shown significant effectiveness in weight loss and blood sugar control compared to a placebo, paving the way for a potential application to the U.S. Food and Drug Administration (FDA) in the coming months. A decision regarding its approval is anticipated in 2024.
Impressive Trial Results
The results come from the ATTAIN-2 study, which involved over 1,600 participants from ten different countries. These individuals were either classified as obese or overweight and had type 2 diabetes. Participants were randomly assigned to receive either a placebo or one of three different doses of orforglipron, taken once daily. The trial lasted for a period of 72 weeks.
The findings revealed that those taking orforglipron experienced more substantial weight loss than those receiving the placebo. Specifically, those on the highest dose lost, on average, about 10% of their baseline body weight, compared to an average loss of just 2.5% in the placebo group. Additionally, the drug performed well on secondary endpoints, with roughly one-third of participants on the highest dose achieving a weight loss of at least 15%.
A Step Forward in Obesity Treatment
Eli Lilly’s orforglipron could represent a significant advancement in obesity treatments, as it would be the first next-generation GLP-1 oral medication for weight management if approved. Current options include only one GLP-1 pill, Rybelsus, developed by Novo Nordisk, which treats type 2 diabetes.
While the prospect of orforglipron is exciting, it may face tough competition. In earlier trials, orforglipron produced an average weight loss of 12.4%, which is slightly less effective than the 14% weight loss reported for Novo Nordisk’s injectable Wegovy. Furthermore, Novo Nordisk is expected to submit a higher-dose version of its oral semaglutide for FDA approval later this year, which could attract a significant portion of the market.
Despite these challenges, many patients may prefer the convenience of a daily pill over a weekly injection, even if the effectiveness is marginally lower. The production of an oral medication is also likely to be more straightforward than injectable therapies, potentially mitigating the shortages that have plagued existing GLP-1 medications.
As Eli Lilly moves forward to seek regulatory approval, the company aims to address the needs of patients eagerly awaiting new treatment options. “With these positive data in hand, we are moving with urgency toward global regulatory submissions to potentially meet the needs of patients who are waiting,” said Kenneth Custer, Executive Vice President of Eli Lilly and President of Lilly Cardiometabolic Health.
With orforglipron’s promising trial outcomes, the landscape of obesity treatment is poised for change. However, it is clear that the competition among weight-loss medications will continue to intensify in the years ahead.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle