Health
Moderna’s Updated COVID-19 Vaccine Shows Promising Results in Trials

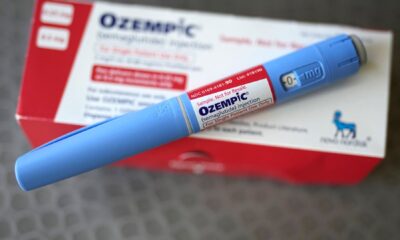
Moderna has announced promising results for its updated COVID-19 vaccine, targeting the LP.8.1 variant. Preliminary analysis indicates that the 2025-2026 formula of Spikevax has generated a more than eight-fold increase in LP.8.1-neutralizing antibodies across various age groups. This development reinforces earlier preclinical data and supports the recent approval of the vaccine by the U.S. Food and Drug Administration (FDA).
The results stem from clinical findings that reflect the vaccine’s enhanced efficacy against this emerging variant. The increase in neutralizing antibodies suggests that the updated formulation significantly boosts the immune response, offering potential protection for individuals receiving the vaccination.
Supporting Evidence and Regulatory Approval
The data presented by Moderna aligns with the company’s ongoing commitment to adapt its vaccine in response to evolving COVID-19 variants. The findings not only validate the scientific approach taken during the vaccine’s development but also highlight the importance of ongoing research in the fight against the pandemic.
According to the company, the clinical trials involved diverse age groups, ensuring that the results are applicable across the population. This broad applicability is crucial, as different age demographics often exhibit varying immune responses. The strong immune response recorded is a positive indicator for public health, especially in light of the continuing presence of COVID-19 variants.
The FDA’s approval of the 2025-2026 Spikevax formula marks a significant step forward. The agency’s rigorous evaluation process ensures that the vaccine meets stringent safety and efficacy standards before being made available to the public. With this approval, Moderna aims to enhance vaccination efforts and protect against the LP.8.1 variant, which has been identified as a concern in various regions.
Future Implications for Public Health
As the world continues to navigate the challenges posed by COVID-19, updates to vaccines like Spikevax are essential. The increasing prevalence of variants underscores the need for vaccines that can effectively counteract these mutations. With the results from this preliminary analysis, Moderna is positioned to play a pivotal role in advancing global vaccination strategies.
Healthcare professionals and policymakers will closely monitor these developments as they plan for future vaccination campaigns. The enhanced immune response observed in clinical trials could lead to increased confidence in vaccine uptake, ultimately contributing to the control of COVID-19 transmission.
In conclusion, the promising results from Moderna’s updated vaccine against the LP.8.1 variant represent a significant advancement in the ongoing battle against the pandemic. As further data becomes available, the healthcare community will be better equipped to respond to the evolving landscape of COVID-19, ensuring that populations remain protected against emerging threats.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle