Health
FDA Expands Use of Addyi Pill for Women Over 65
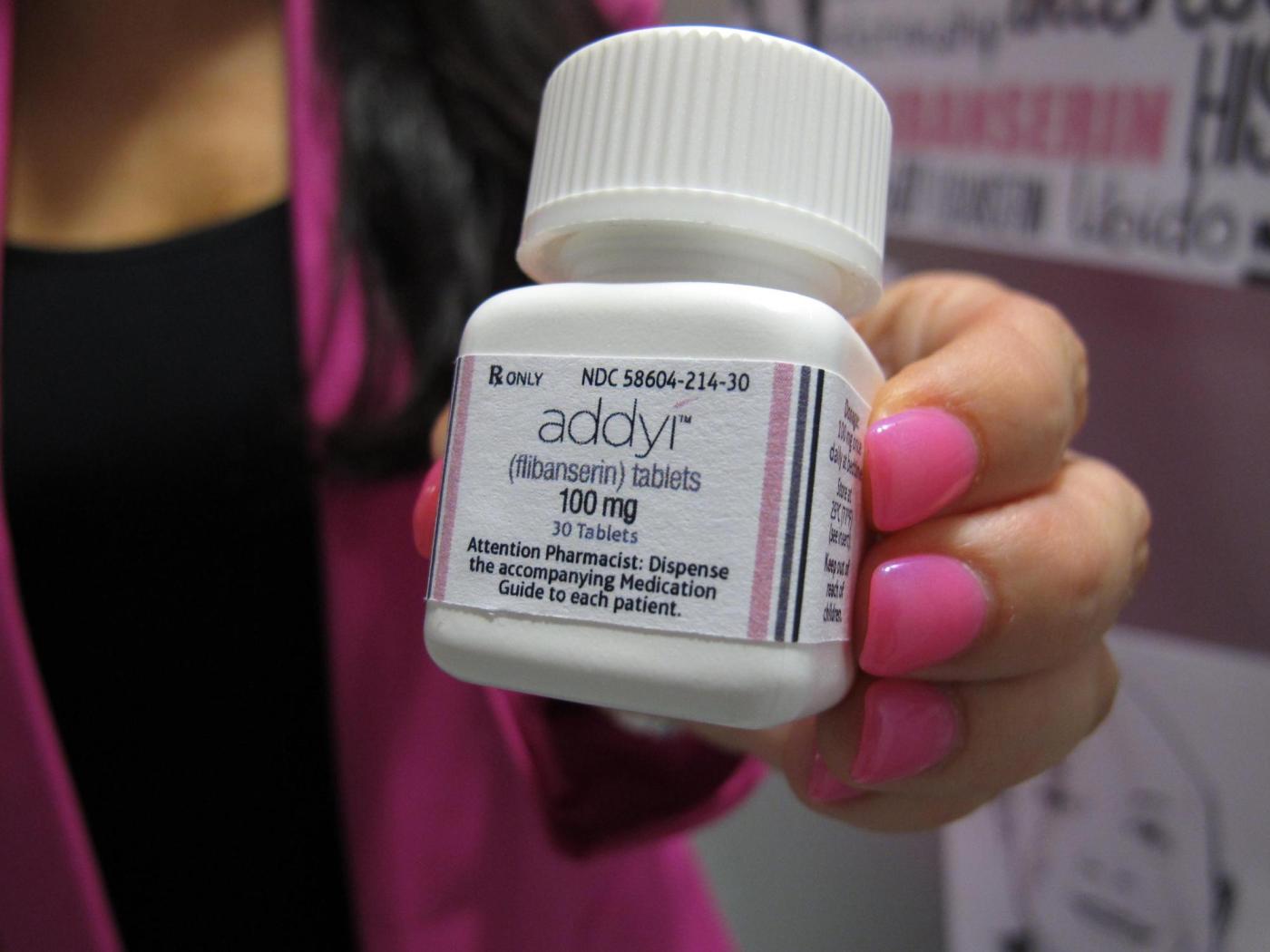
U.S. health officials have expanded the approval of the libido-boosting pill, Addyi, to include women over the age of 65 who have undergone menopause. The announcement from the Food and Drug Administration (FDA) on March 4, 2024, marks a significant development in the treatment options available for women experiencing low sexual desire.
Initially approved in 2015 for premenopausal women suffering from emotional stress related to low libido, Addyi is now accessible to a broader demographic. The change comes after ongoing discussions regarding women’s sexual health and the challenges faced by older women in this area. The FDA’s decision reflects a growing recognition of the complexities surrounding female sexual health.
Concerns and Limitations of Addyi
Manufactured by Sprout Pharmaceuticals, Addyi has faced criticism for its side effects, which can include dizziness and nausea. It carries a boxed warning—the FDA’s most serious type—alerting users to the potential risks of combining the medication with alcohol, which can lead to dangerously low blood pressure and fainting.
Despite its intended purpose, sales of Addyi have remained low. The pill works by influencing brain chemicals that affect both mood and appetite, but the challenges of side effects and a complicated approval history have limited its market impact. In 2019, the FDA approved a second treatment for low female libido, an on-demand injection targeting a different neurological mechanism.
Broader Implications for Women’s Health
The medical condition associated with low sexual desire is known as hypoactive sexual desire disorder (HSDD). This condition has been recognized since the 1990s and is estimated to affect a significant number of women in the United States. The approval of Addyi is seen as a step toward addressing a long-neglected aspect of women’s health.
According to surveys, many women experience diminished libido after menopause due to hormonal changes. Diagnosing HSDD can be complex, as healthcare providers must consider various factors, including relationship issues, medical conditions, and psychological factors, before prescribing treatment.
Critics of the diagnosis argue that low sexual desire should not necessarily be classified as a medical issue. Nevertheless, advocates for women’s sexual health, including Cindy Eckert, CEO of Sprout Pharmaceuticals, emphasize the importance of improving understanding and prioritization of this issue. Eckert stated that the FDA’s recent approval reflects a decade of effort to bring attention to women’s sexual health needs.
The approval process for Addyi was contentious, with the drug facing rejection from the FDA twice prior to its eventual approval. These rejections were based on concerns about its modest effectiveness and potential side effects. However, a lobbying campaign by Sprout and its supporters reframed the discussion around women’s sexual health as a matter of rights, which contributed to the eventual approval.
As the conversation around women’s health continues to evolve, the expansion of Addyi’s approval signals a growing acknowledgment of the complexities surrounding female libido and the need for more comprehensive treatment options.
-

 Technology5 months ago
Technology5 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Technology2 weeks ago
Technology2 weeks agoOpenAI to Implement Age Verification for ChatGPT by December 2025
-

 Health3 months ago
Health3 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Health3 months ago
Health3 months agoAnalysts Project Stronger Growth for Apple’s iPhone 17 Lineup
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology3 months ago
Technology3 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology2 months ago
Technology2 months agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology5 months ago
Technology5 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology5 months ago
Technology5 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health5 months ago
Health5 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle5 months ago
Lifestyle5 months agoBelton Family Reunites After Daughter Survives Hill Country Floods