Health
EU Approves Tislelizumab for High-Risk Lung Cancer Patients
The European Commission (EC) has granted approval for tislelizumab (Tevimbra) as a combination treatment for adults with resectable non-small cell lung cancer (NSCLC) at high risk of recurrence. This decision allows the use of tislelizumab alongside platinum-containing chemotherapy as a neoadjuvant therapy, followed by tislelizumab monotherapy as adjuvant therapy. The approval is based on findings from the phase 3 RATIONALE-315 trial (NCT04379635), which demonstrated a statistically significant improvement in overall survival (OS) for patients receiving the tislelizumab regimen compared to those receiving chemotherapy plus placebo.
Data from the trial will be presented in September 2025 at the IASLC World Conference on Lung Cancer. Mariano Provencio, MD, PhD, head of the Medical Oncology Department at Hospital Universitario Puerta de Hierro and professor at Universidad Autónoma de Madrid, emphasized the importance of this approval, stating, “Patients with resectable NSCLC still face alarmingly high recurrence rates. The RATIONALE-315 results confirm that starting tislelizumab in the neoadjuvant phase and continuing after surgery has proven to be a powerful approach to improve outcomes for these patients.”
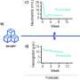
The interim analysis of RATIONALE-315 indicated that patients treated with tislelizumab experienced a statistically significant improvement in event-free survival (EFS), with a hazard ratio (HR) of 0.56 (95% CI, 0.40-0.79; P = .0003). Furthermore, the combination of tislelizumab and chemotherapy achieved a major pathological response (MPR) rate of 56.2%, compared to 15.0% for chemotherapy plus placebo (difference, 41.1%; 95% CI, 33.2%-49.1%; P < .0001). The pathological complete response (pCR) rates were also notable, with 40.7% for tislelizumab versus 5.7% for placebo (difference, 35.0%; 95% CI, 27.9%-42.1%; P < .0001). Regarding safety, treatment-related adverse effects of grade 3 or higher occurred in 72.1% of patients in the tislelizumab arm, compared to 66.4% in the placebo group. Serious treatment-related adverse effects were reported at rates of 15.5% for the tislelizumab group and 8.0% for placebo. The most frequently reported grade 3 or 4 adverse effects in the experimental arm included decreased neutrophil and white blood cell counts.
The RATIONALE-315 trial was a randomized, double-blind, placebo-controlled study that enrolled participants aged 18 and older with histologically confirmed stage II or IIIA NSCLC, eligible for R0 resection. Key inclusion criteria included measurable disease as per RECIST 1.1 criteria and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients who had received prior therapy for their lung cancer, including chemotherapy or radiotherapy, or those with known EGFR mutations or ALK translocations were excluded. Participants were randomly assigned to receive tislelizumab with either cisplatin or carboplatin and paclitaxel or pemetrexed as neoadjuvant therapy, followed by tislelizumab alone in the adjuvant phase, or placebo with the same chemotherapy regimen.
The trial’s dual primary endpoints were MPR rate and EFS, while secondary endpoints included OS, pCR rate, objective response rate, disease-free survival, safety, and quality of life.
Mark Lanasa, MD, PhD, chief medical officer of Solid Tumors at BeOne Medicines, remarked on the significance of the EC’s approval, stating, “Delivering a statistically significant OS benefit—a critical endpoint in oncology studies—alongside the EC’s approval of tislelizumab in perioperative resectable NSCLC marks a pivotal moment for patients and physicians. As only the second PD-1 inhibitor to demonstrate an OS benefit in this setting, tislelizumab is poised to reshape lung cancer treatment in Europe.”
With this new treatment option now available in the EU, there is hope for improved outcomes in a patient population that has long faced high rates of recurrence post-surgery. The full impact of this breakthrough therapy will be further elucidated once the comprehensive data from the RATIONALE-315 trial is unveiled at the upcoming conference.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle