Health
Discovery of RNA Molecule Links Kidney Function to Autoimmune Disease
A collaborative research effort led by scientists at the University Hospital Bonn and the University of Bonn has unveiled how a small RNA molecule in the kidney can activate a mutated immune receptor, leading to severe autoimmune diseases. The study, published in the journal Science Immunology, sheds light on a critical mechanism that transforms the body’s immune response into a self-destructive process.
The research team, including partners from Nanyang Technological University Singapore and the University Hospital Würzburg, focused on a specific mutation in the immune receptor known as RIG-I. This receptor plays a vital role in the innate immune system by recognizing viral RNA and activating antiviral defenses. However, mutations in the genetic material can lead RIG-I to become hypersensitive, causing it to mistakenly identify the body’s own RNA as harmful invaders.
In their experiments, researchers found that mice with the RIG-I E373A mutation developed lupus-like nephritis, a severe kidney inflammation that can be fatal. Unlike classic lupus, where inflammation results from immune complex deposits, the disease in these mice stemmed from direct kidney inflammation due to the malfunctioning RIG-I receptor.
How RNA Triggers Autoimmune Responses
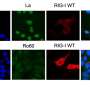
Further investigations revealed that a short, non-coding RNA called Y-RNA, which is abundantly produced in the kidney, binds directly to the mutated RIG-I. This binding initiates an abnormal activation of the receptor. According to Prof. Hiroki Kato, Director of the Institute for Cardiovascular Immunology at UKB, “Y-RNA acts like a false alarm for the mutated RIG-I receptor, especially in kidney cells.” This local immune malfunction leads to significant inflammation similar to that observed in human lupus nephritis.
Through advanced molecular and structural analyses, the research team demonstrated that the RIG-I E373A mutant engages with Y-RNA in an atypical manner, activating the receptor even in the absence of viral infection. This abnormal activation prompts kidney cells to produce elevated levels of interferons and chemokines, drawing immune cells to the area and triggering inflammation.
Potential Therapeutic Targets Identified
Interestingly, the researchers also identified a potential therapeutic target in their study. They found that blocking the CCR2 signaling pathway, responsible for recruiting monocytes – a type of white blood cell – significantly reduced kidney inflammation in the affected mice. This discovery could lead to new treatment options for autoimmune diseases associated with RIG-I mutations.
Mutations in RIG-I have been linked to rare hereditary diseases, including Singleton-Merten syndrome and systemic lupus erythematosus. The insights gained from this research offer a clearer understanding of how such mutations can specifically damage organs like the kidney and highlight possibilities for targeted therapies aimed at inhibiting the activation of mutated RIG-I or its interacting Y-RNAs.
As the scientific community continues to explore these findings, the potential for developing effective treatments for autoimmune diseases grows stronger, paving the way for better patient outcomes in the future.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle