Health
COVID-19 Vaccine Makers Initiate New Trials for Younger Adults
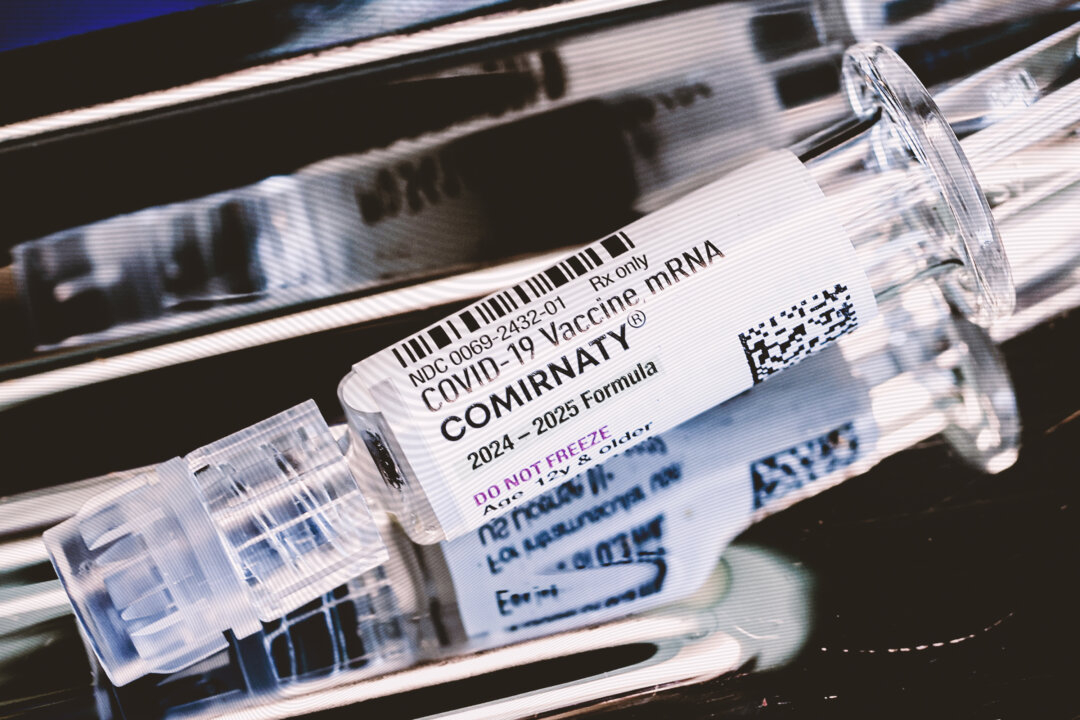
Companies producing COVID-19 vaccines, including Pfizer and Moderna, have announced plans to conduct new clinical trials aimed at evaluating their vaccines in younger populations. This commitment follows the release of official documents that outline the companies’ intentions, ensuring that their products meet safety and efficacy standards for adults aged 50 to 64 who do not have conditions that increase their risk of severe COVID-19.
In a letter dated August 27, 2023, Dr. David Kaslow, an official with the U.S. Food and Drug Administration (FDA), confirmed that Pfizer and its partner BioNTech will conduct a randomized, double-blind, placebo-controlled trial. This study will assess the safety and effectiveness of their COVID-19 vaccine in the specified age group, which is critical given the evolving landscape of the pandemic and the ongoing need for updated data.
Similarly, Moderna has also committed to a new trial. The company will conduct a randomized, observer-blind, placebo-controlled study to evaluate the safety of its two COVID-19 vaccines in the same demographic. This represents a significant step towards ensuring that vaccine formulations remain relevant and effective as populations continue to face the challenges posed by COVID-19.
The decision to initiate these trials reflects the ongoing commitment of vaccine manufacturers to adapt to changing health needs and to ensure the safety of their products across diverse age groups. As the pandemic persists, monitoring the responses of different populations to existing vaccines will be crucial for public health strategies.
Both trials are expected to yield important data that can inform future vaccination recommendations and public health policies. The results will not only help determine the safety profiles of these vaccines in younger adults but also contribute to broader understanding of the immune response elicited by the vaccines in this age group.
As vaccination efforts continue worldwide, the commitment from Pfizer, BioNTech, and Moderna to conduct these trials is a vital part of the ongoing response to COVID-19, ensuring that effective vaccines remain available to protect populations at risk. This proactive approach highlights the importance of rigorous testing and transparency in the development of vaccines, which remains critical in the fight against the global pandemic.
-

 Technology4 months ago
Technology4 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology3 weeks ago
Technology3 weeks agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology4 months ago
Technology4 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology4 months ago
Technology4 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle4 months ago
Lifestyle4 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride
-

 Technology4 weeks ago
Technology4 weeks agoDiscover the Best Wireless Earbuds for Every Lifestyle