Health
Alzinova’s ALZ-101 Vaccine Enters Phase II Trials for Alzheimer’s
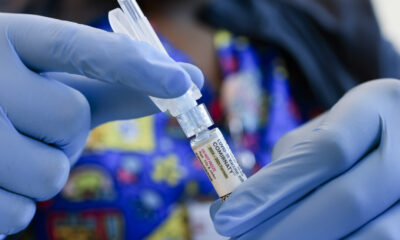
The Swedish biopharmaceutical company Alzinova has received approval from the U.S. Food and Drug Administration (FDA) to advance its Alzheimer’s vaccine candidate, ALZ-101, into a Phase II clinical study. This significant milestone follows positive results from a prior Phase Ib trial and marks a crucial step in the development of a potential treatment for Alzheimer’s disease.
ALZ-101 aims to stimulate the immune system to produce antibodies against toxic amyloid-beta (Aβ) oligomers, which are known to contribute to the progression of Alzheimer’s. The FDA’s approval of Alzinova’s Investigational New Drug (IND) application confirms that the company is ready to undertake the next stage of clinical evaluation.
Tord Labuda, CEO of Alzinova, expressed enthusiasm about the IND approval, stating, “The FDA’s approval of our IND is a decisive confirmation of our readiness to advance ALZ-101 into the next stage of development. The approval gives us the green light to initiate the Phase II study in the United States and to build on the promising results from Phase Ib.”
Phase II Study Details
The upcoming Phase II clinical study is designed as a multicenter trial that will involve approximately 240 patients diagnosed with early Alzheimer’s disease. The trial will assess the safety, tolerability, and efficacy of ALZ-101. Following the administration of the vaccine, participants will undergo a series of follow-up visits to evaluate both primary and secondary endpoints. Primary endpoints will focus on cognitive tests, while secondary endpoints will include further assessments of safety and tolerability.
The study will be conducted in collaboration with Worldwide Clinical Trials, a contract research organization that will help facilitate the trial across various clinical sites in the United States. This extensive approach allows for a broader evaluation of the vaccine’s effectiveness and safety profile.
Alzinova previously conducted a first-in-human clinical study that investigated the safety and immunogenicity of ALZ-101. Results from part A of the Phase Ib clinical trial, reported in November 2023, provided favorable data, reinforcing the company’s confidence in advancing the vaccine to a larger patient population.
Progress and Future Implications
The IND approval signifies that Alzinova has met all regulatory requirements regarding the study design, safety data, and manufacturing processes for ALZ-101. This advancement not only validates the company’s previous research but also brings it closer to its goal of making ALZ-101 available to patients struggling with Alzheimer’s.
Alzheimer’s disease currently affects millions globally, and the need for effective treatments remains urgent. By targeting the underlying mechanisms of the disease, Alzinova’s vaccine candidate may offer hope for improved management of this debilitating condition. The Phase II study is anticipated to provide critical insights into the viability of ALZ-101 as a therapeutic option.
As the clinical trial progresses, the outcomes may significantly influence the landscape of Alzheimer’s treatment, potentially paving the way for new immunotherapy approaches targeting the disease’s root causes.
-

 Technology5 months ago
Technology5 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology1 month ago
Technology1 month agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology5 months ago
Technology5 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology5 months ago
Technology5 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle5 months ago
Lifestyle5 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology4 months ago
Technology4 months agoHarmonic Launches AI Chatbot App to Transform Mathematical Reasoning
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride