Science
New Catalyst Boosts Efficiency of Electrochemical Methane Conversion
Recent advancements in electrochemical technology have led to a significant breakthrough in the conversion of methane, a key component of natural gas, into valuable chemicals. Researchers from the Sinopec Beijing Research Institute of Chemical Industry and Tsinghua University have developed an innovative catalyst that enhances both the efficiency and stability of the electrochemical oxidative coupling of methane (EOCM) process. This development could have profound implications for the energy and chemical sectors.
Traditional oxidative coupling of methane (OCM) methods have struggled with issues such as low product selectivity and high operational costs, particularly due to the reliance on pure oxygen oxidants. The newly introduced EOCM technology overcomes these challenges by separating methane from oxidants and managing oxygen ion migration more effectively. Yet, the stability of catalysts for reactive oxygen species has limited the yield and selectivity of C2 products.
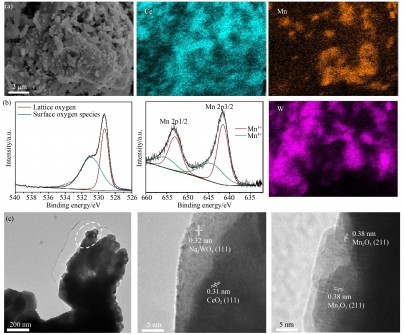
In response to these limitations, a team led by Yifeng Li and Yu Bo has created a novel electrode using a perovskite material, La0.6Sr0.4MnO3–δ (LSM), paired with a composite of Ce-Mn-W oxides. The support material, (Ce0.90Gd0.10)O1.95 (GDC), plays a crucial role in stabilizing reactive oxygen species during the oxygen evolution reaction (OER).
By optimizing the interaction between these materials, the researchers achieved remarkable results. Under conditions of 850 °C, the C2 product selectivity of the CMW@GDC/LSM composite electrode reached a maximum of 80.3%, with a yield of 313 μL/min/cm2 at a current density of 600 mA/cm2. This performance significantly surpasses that of either the pure LSM or single CMW catalysts.
The design of the composite electrode not only stabilizes reactive oxygen species but also enhances the adsorption capacity of methane, thereby facilitating the activation of C-H bonds and the formation of C-C bonds. This advancement addresses critical hurdles faced by traditional catalysts, achieving a balance between selectivity and activity.
Furthermore, the electrode demonstrated substantial stability, maintaining approximately 70% C2 selectivity over 5500 seconds of continuous operation without significant deactivation. This robustness is attributed to the synergistic effects between the GDC support, which stabilizes O2 2- species and reduces their formation free energy, and LSM, which provides excellent electronic conductivity and OER activity.
This research, published in November 2023, represents a pivotal step toward the precise regulation of reactive oxygen species through engineered material interfaces. The implications extend beyond enhanced methane conversion; the technology couples methane conversion with CO2 electrolysis, improving energy conversion efficiency and promoting carbon cycling via cathodic CO2 reduction. This innovative approach offers a promising solution for developing low-carbon energy systems, addressing both environmental concerns and energy needs in a sustainable manner.
In summary, the collaborative efforts of Yifeng Li, Yu Bo, and their teams mark a significant advancement in the quest for efficient methane conversion technologies, potentially reshaping the landscape of chemical manufacturing and energy production.
-

 Technology5 months ago
Technology5 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology1 month ago
Technology1 month agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology5 months ago
Technology5 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology5 months ago
Technology5 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle5 months ago
Lifestyle5 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology4 months ago
Technology4 months agoHarmonic Launches AI Chatbot App to Transform Mathematical Reasoning
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride