Health
Rush University Launches Blood Test for Early Cancer Detection
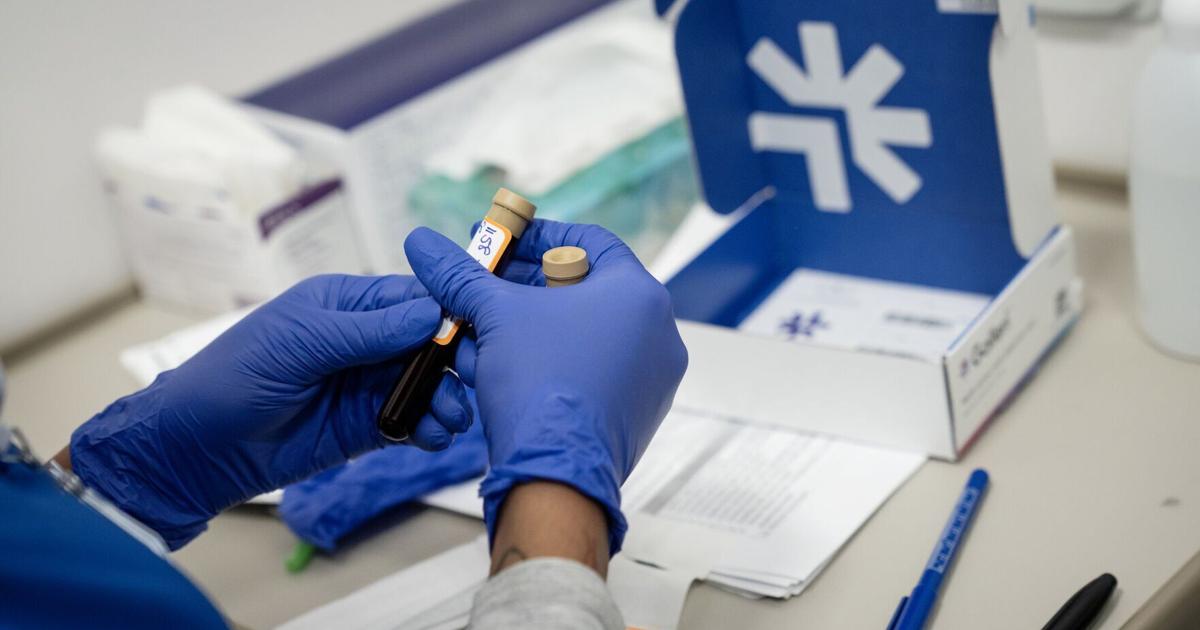
A new blood test called Galleri is now being offered widely at the Rush University System for Health in Chicago. This test aims to detect over 50 types of cancer at an early stage, potentially improving treatment outcomes. While the test has gained traction among some health systems, others remain cautious, citing concerns about its approval status and effectiveness.
The story of David Welter, a 61-year-old resident of Elkhart, Indiana, highlights the potential benefits of early detection. After a positive result from Galleri, Welter underwent follow-up tests that confirmed cancer in his throat. He successfully completed radiation and chemotherapy in 2023 and is now cancer-free. “Without that test… it might not have appeared for another year or more,” Welter remarked, emphasizing the importance of early detection.
Despite its promise, Galleri has not yet received approval from the U.S. Food and Drug Administration (FDA), and most health insurance plans do not cover its cost, which is approximately $749 out-of-pocket at Rush. Dr. Lisa Stempel, director of the high-risk cancer screening program at Rush, explained that the decision to offer the test was driven by its potential life-saving benefits and positive results from clinical trials. “The goal of all screening is to find cancer early when we can treat it,” she stated.
The Galleri test detects DNA released by cancer cells into the bloodstream, identifying signals shared by multiple cancer types and indicating their possible location. While a positive result suggests further testing, such as CT or PET scans, it does not confirm a diagnosis. According to a study by Grail, the company behind Galleri, there is a 43% likelihood that a positive result indicates cancer. Additionally, the test has a false positive rate of 0.5%, meaning that approximately one in every 200 individuals without cancer may still receive a positive result.
The test is available by prescription and is recommended annually for individuals at higher risk of cancer, particularly those aged 50 and older. Rush also extends the offering to younger adults with risk factors such as a family history of cancer, smoking, diabetes, or obesity. If a patient receives a positive result but subsequent tests do not confirm cancer, Grail provides a second test at no charge.
Welter’s experience with the Galleri test exemplifies its potential impact. After his initial positive result, follow-up tests did not reveal cancer. However, a second Galleri test, conducted six months later, confirmed the presence of cancer, leading to timely treatment. Welter has shared his journey with Rush doctors to support their training in offering the test to patients.
Not all healthcare providers, however, share Rush’s enthusiasm. While some health systems in the U.S. have begun offering Galleri, others, including Northwestern Medicine, have expressed skepticism. A spokesperson stated that the technology is “not sufficiently sensitive and specific enough for us to use this as a screening tool at this time.” Concerns also arise regarding the potential for increased healthcare disparities due to the test’s cost.
Feighanne Hathaway, a genetic counselor at UChicago Medicine, voiced multiple concerns about Galleri, including its efficacy in detecting early-stage cancers and the possibility that patients might forgo routine screenings based on negative results. Grail’s data indicates that 48% of confirmed cancers detected by the test were in stages 1 or 2, and it doubled the number of cancers identified when added to standard screening protocols. Nonetheless, Hathaway emphasized the importance of further research before widespread adoption.
Clinical trials involving over 380,000 participants have been conducted to assess Galleri’s efficacy, including a recent trial in the United Kingdom with 140,000 participants. Grail is sponsoring an additional trial aimed at enrolling 50,000 Medicare beneficiaries to evaluate whether Galleri can reduce late-stage cancer diagnoses. Although Galleri has not yet received FDA approval, it has been granted breakthrough device designation by the agency, which facilitates faster development and review for promising medical technologies.
Dr. Joshua Ofman, president of Grail, articulated the significance of Galleri, stating that it offers the opportunity to detect cancers that currently lack recommended screening methods. “We can look for all the other cancers that are taking people’s lives,” he noted, highlighting the potential for early detection to improve survival rates.
Patients like Peter Crowell, 65, have already taken the initiative to undergo testing. Crowell, who has a family history of cancer, proactively sought Galleri after discussing it with his primary care physician. While he wishes insurance would cover the cost, he is prepared to pay for the test using a flexible spending account, considering the potential benefits to his health.
As awareness of Galleri grows, healthcare professionals at Rush, like nurse practitioner Maggie Hornung, are eager to offer the test. Hornung, who has family members affected by cancer, recognizes the importance of early detection. “To me, $749 is a small cost compared to what things could be,” she remarked.
In summary, while the Galleri test presents a promising advancement in early cancer detection, its widespread adoption faces scrutiny. Ongoing clinical trials and research will be crucial in determining its long-term efficacy and viability as a standard screening tool.
-

 Lifestyle1 week ago
Lifestyle1 week agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Education2 weeks ago
Education2 weeks agoWinter Park School’s Grade Drops to C, Parents Express Concerns
-

 Technology2 weeks ago
Technology2 weeks agoByteDance Ventures into Mixed Reality with New Headset Development
-

 Technology2 weeks ago
Technology2 weeks agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Lifestyle2 weeks ago
Lifestyle2 weeks agoNew Restaurants Transform Minneapolis Dining Scene with Music and Flavor
-

 Technology6 days ago
Technology6 days agoMathieu van der Poel Withdraws from Tour de France Due to Pneumonia
-

 Technology2 weeks ago
Technology2 weeks agoGlobal Market for Air Quality Technologies to Hit $419 Billion by 2033
-

 Health2 weeks ago
Health2 weeks agoSudden Vision Loss: Warning Signs of Stroke and Dietary Solutions
-

 Technology2 weeks ago
Technology2 weeks agoAnalysts Highlight Top 5 Altcoin Presales Ahead of Market Surge
-

 Technology2 weeks ago
Technology2 weeks agoTrump Faces Internal Struggles Over Epstein Files Handling
-

 Technology2 weeks ago
Technology2 weeks agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health2 weeks ago
Health2 weeks agoBacteria Navigate Gut Risks for Nutrients, New Study Reveals