Health
Clinical Trial Shows Evolocumab Reduces Heart Attack Risk by 25%
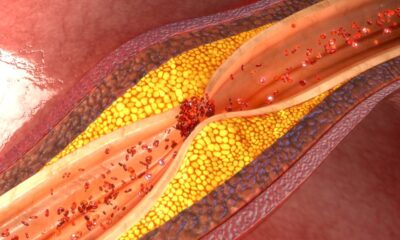
A significant clinical trial conducted by researchers from Mass General Brigham has revealed that the drug evolocumab can substantially decrease the risk of first heart attacks and strokes in high-risk adults. Results were presented at the American Heart Association Scientific Sessions and published in the New England Journal of Medicine on March 15, 2025.
According to Dr. Erin Bohula, a cardiologist at the Mass General Brigham Heart and Vascular Institute, “The results of this trial offer hope for preventing a first heart attack, stroke, or other cardiovascular event in patients who are at high risk.” This trial emphasizes the importance of preventive strategies in healthcare, showcasing a commitment to advancing patient care through rigorous clinical research.
The VESALIUS-CV trial, officially known as The Effect of EVolocumab in PatiEntS at High CArdiovascuLar RIsk WithoUt Prior Myocardial Infarction or Stroke, is a phase 3 study focusing on individuals with high cardiovascular risk. This trial is particularly noteworthy as it is the first to investigate the preventive effects of evolocumab in patients who have not previously experienced a heart attack or stroke.
Participants in the trial included 12,257 adults enrolled worldwide, all of whom had atherosclerosis or diabetes and LDL cholesterol levels above 90 mg/dL. Importantly, candidates with a history of heart attack or stroke were excluded. Participants were randomly assigned to receive either evolocumab (140 mg administered biweekly) or a placebo, alongside their standard care regimen, and were monitored for a median of 4.6 years.
During the study, the results indicated that 336 patients (6.2%) in the evolocumab group experienced major adverse cardiovascular events, compared to 443 patients (8.0%) in the placebo group. This translates to a 25% reduction in risk for events such as coronary heart disease death, myocardial infarction, or ischemic stroke. Furthermore, those receiving evolocumab experienced a 36% reduction in heart attacks compared to their counterparts receiving the placebo.
While there was a nominally lower rate of death among those taking evolocumab (7.9% versus 9.7% in the placebo group), the authors noted that the majority of participants were on high-intensity statins or other cholesterol-lowering medications, although some were on less intensive treatments or none at all. The findings were consistent across varying treatment backgrounds.
Despite the promising outcomes, the trial’s demographic makeup, predominantly white participants, raises questions about the generalizability of the results to diverse populations.
Dr. Marc S. Sabatine, Chair of the TIMI Study Group, remarked on the results, stating, “In VESALIUS-CV, patients in the evolocumab arm achieved LDL-C levels of around 40 mg/dL. I believe that is what we should be targeting in these patients.”
The implications of this trial are significant, highlighting the potential of evolocumab as a vital tool in preventing cardiovascular events in high-risk individuals. As researchers continue to explore the nuances of these findings, the hope is to further improve patient outcomes and quality of life through advanced therapeutic strategies.
-

 Technology5 months ago
Technology5 months agoDiscover the Top 10 Calorie Counting Apps of 2025
-

 Health2 months ago
Health2 months agoBella Hadid Shares Health Update After Treatment for Lyme Disease
-

 Health3 months ago
Health3 months agoErin Bates Shares Recovery Update Following Sepsis Complications
-

 Technology4 months ago
Technology4 months agoDiscover How to Reverse Image Search Using ChatGPT Effortlessly
-

 Technology1 month ago
Technology1 month agoDiscover 2025’s Top GPUs for Exceptional 4K Gaming Performance
-

 Technology2 months ago
Technology2 months agoElectric Moto Influencer Surronster Arrested in Tijuana
-

 Technology5 months ago
Technology5 months agoMeta Initiates $60B AI Data Center Expansion, Starting in Ohio
-

 Technology5 months ago
Technology5 months agoRecovering a Suspended TikTok Account: A Step-by-Step Guide
-

 Health4 months ago
Health4 months agoTested: Rab Firewall Mountain Jacket Survives Harsh Conditions
-

 Lifestyle5 months ago
Lifestyle5 months agoBelton Family Reunites After Daughter Survives Hill Country Floods
-

 Technology4 months ago
Technology4 months agoHarmonic Launches AI Chatbot App to Transform Mathematical Reasoning
-

 Technology3 months ago
Technology3 months agoUncovering the Top Five Most Challenging Motorcycles to Ride